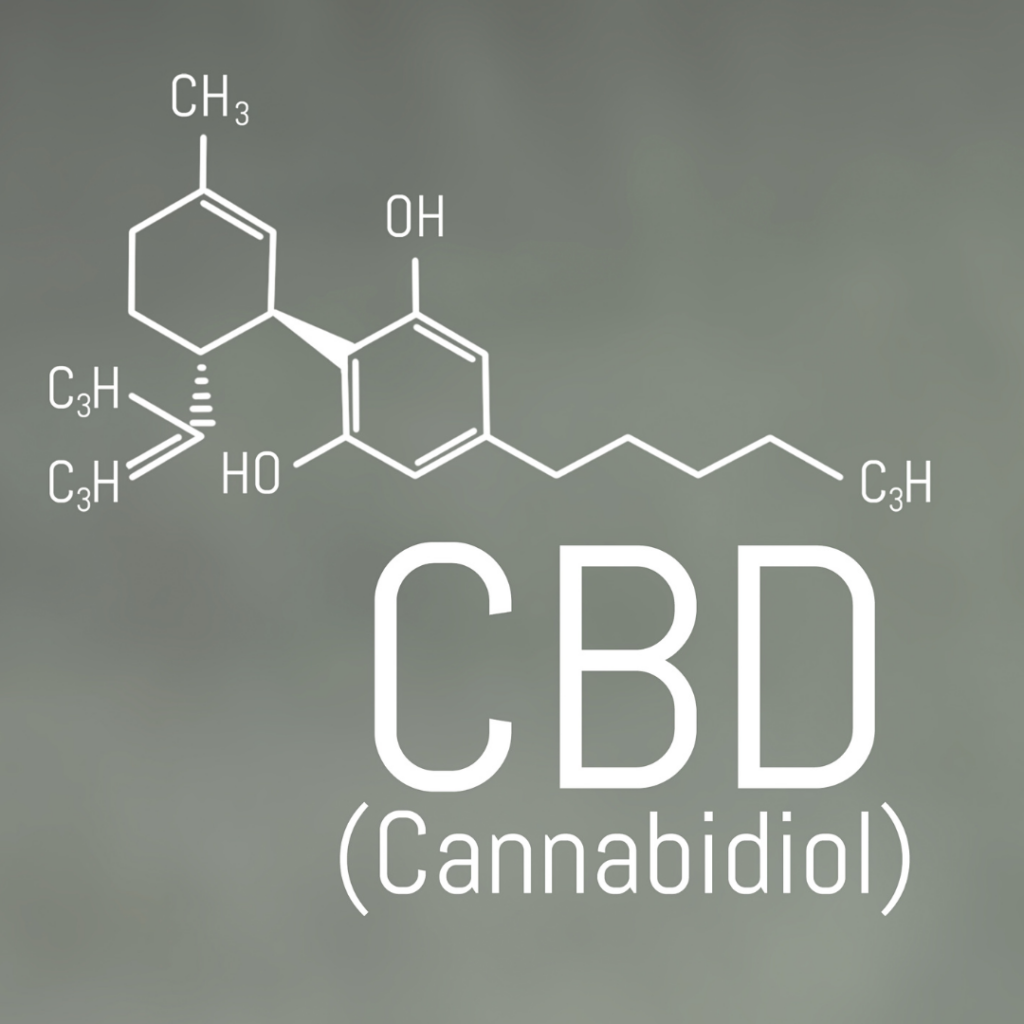
Since 2021, CBD (cannabidiol) and CBD-containing products have been subject to the EU Novel Food Regulation (EU) 2015/2283 when seeking authorization for the European market as a novel food. In recent years, cannabidiol, commonly known as CBD, has emerged as a prominent player in the health and wellness industry. Touted for its potential therapeutic properties,
CBD: Unraveling the Health Benefits and Regulatory Landscape